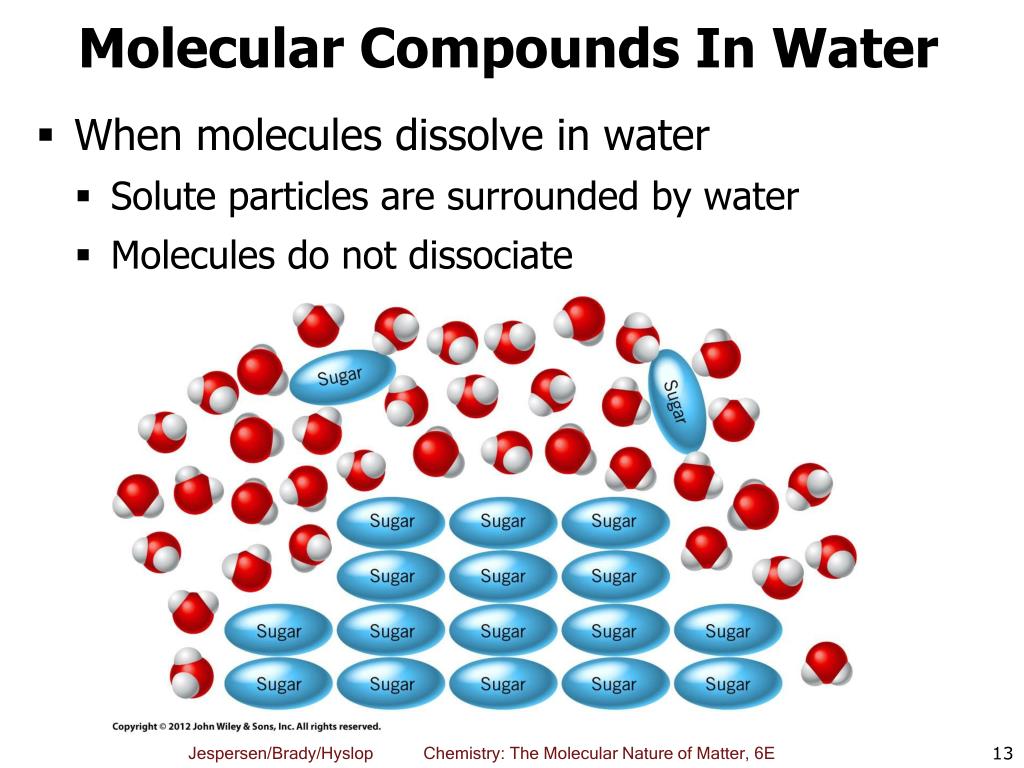
What Compound Is Insoluble In Water . while many compounds are partially or mostly insoluble, there is no substance that is completely insoluble in water, meaning that it can't dissolve at all. for example, ethanol is soluble in water. You will see in the. However, to predict whether the compound will dissolve or not, we need to. In insoluble chemical does not dissolve in the solvent. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. our article on solubility explains how ionic compounds dissolve in water to form a solution. Nonelectrolytes are substances that do not produce ions when. The compounds are dissolved in. substances that dissolve in water to yield ions are called electrolytes.
from www.slideserve.com
while many compounds are partially or mostly insoluble, there is no substance that is completely insoluble in water, meaning that it can't dissolve at all. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. In insoluble chemical does not dissolve in the solvent. substances that dissolve in water to yield ions are called electrolytes. our article on solubility explains how ionic compounds dissolve in water to form a solution. Nonelectrolytes are substances that do not produce ions when. The compounds are dissolved in. However, to predict whether the compound will dissolve or not, we need to. You will see in the. for example, ethanol is soluble in water.
PPT Chapter 5 Molecular View of Reactions in Aqueous Solutions Part I
What Compound Is Insoluble In Water You will see in the. You will see in the. The compounds are dissolved in. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. In insoluble chemical does not dissolve in the solvent. substances that dissolve in water to yield ions are called electrolytes. However, to predict whether the compound will dissolve or not, we need to. Nonelectrolytes are substances that do not produce ions when. while many compounds are partially or mostly insoluble, there is no substance that is completely insoluble in water, meaning that it can't dissolve at all. for example, ethanol is soluble in water. our article on solubility explains how ionic compounds dissolve in water to form a solution.
From www.chegg.com
Solved Part A Which one of the following compounds is What Compound Is Insoluble In Water our article on solubility explains how ionic compounds dissolve in water to form a solution. substances that dissolve in water to yield ions are called electrolytes. for example, ethanol is soluble in water. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. Nonelectrolytes are substances that. What Compound Is Insoluble In Water.
From www.academia.edu
(DOC) Organic compounds insoluble in water bongking naba Academia.edu What Compound Is Insoluble In Water The compounds are dissolved in. our article on solubility explains how ionic compounds dissolve in water to form a solution. In insoluble chemical does not dissolve in the solvent. substances that dissolve in water to yield ions are called electrolytes. Nonelectrolytes are substances that do not produce ions when. However, to predict whether the compound will dissolve or. What Compound Is Insoluble In Water.
From www.slideserve.com
PPT Which of the following is evidence that a chemical reaction has What Compound Is Insoluble In Water The compounds are dissolved in. In insoluble chemical does not dissolve in the solvent. Nonelectrolytes are substances that do not produce ions when. for example, ethanol is soluble in water. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. You will see in the. However, to predict whether. What Compound Is Insoluble In Water.
From socratic.org
Why are metallic compounds insoluble in water? Socratic What Compound Is Insoluble In Water for example, ethanol is soluble in water. In insoluble chemical does not dissolve in the solvent. However, to predict whether the compound will dissolve or not, we need to. while many compounds are partially or mostly insoluble, there is no substance that is completely insoluble in water, meaning that it can't dissolve at all. Nonelectrolytes are substances that. What Compound Is Insoluble In Water.
From study.com
Compound Solubility in Water Overview & Examples Lesson What Compound Is Insoluble In Water while many compounds are partially or mostly insoluble, there is no substance that is completely insoluble in water, meaning that it can't dissolve at all. The compounds are dissolved in. substances that dissolve in water to yield ions are called electrolytes. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout. What Compound Is Insoluble In Water.
From www.slideserve.com
PPT With enough water molecules, a soluble ionic compound is What Compound Is Insoluble In Water You will see in the. The compounds are dissolved in. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. substances that dissolve in water to yield ions are called electrolytes. while many compounds are partially or mostly insoluble, there is no substance that is completely insoluble in. What Compound Is Insoluble In Water.
From www.slideserve.com
PPT Chapter 5 Molecular View of Reactions in Aqueous Solutions Part I What Compound Is Insoluble In Water substances that dissolve in water to yield ions are called electrolytes. our article on solubility explains how ionic compounds dissolve in water to form a solution. for example, ethanol is soluble in water. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. In insoluble chemical does. What Compound Is Insoluble In Water.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation, free download ID6635190 What Compound Is Insoluble In Water In insoluble chemical does not dissolve in the solvent. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. The compounds are dissolved in. Nonelectrolytes are substances that do not produce ions when. while many compounds are partially or mostly insoluble, there is no substance that is completely insoluble. What Compound Is Insoluble In Water.
From www.numerade.com
SOLVED 9.Which of the following compounds is insoluble in water? (2 What Compound Is Insoluble In Water The compounds are dissolved in. Nonelectrolytes are substances that do not produce ions when. substances that dissolve in water to yield ions are called electrolytes. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. while many compounds are partially or mostly insoluble, there is no substance that. What Compound Is Insoluble In Water.
From saylordotorg.github.io
Aqueous Solutions What Compound Is Insoluble In Water The compounds are dissolved in. In insoluble chemical does not dissolve in the solvent. substances that dissolve in water to yield ions are called electrolytes. Nonelectrolytes are substances that do not produce ions when. while many compounds are partially or mostly insoluble, there is no substance that is completely insoluble in water, meaning that it can't dissolve at. What Compound Is Insoluble In Water.
From www.breakingatom.com
Solubility of Elements and Compounds What Compound Is Insoluble In Water In insoluble chemical does not dissolve in the solvent. our article on solubility explains how ionic compounds dissolve in water to form a solution. for example, ethanol is soluble in water. The compounds are dissolved in. Nonelectrolytes are substances that do not produce ions when. when ionic compounds dissolve in water, the ions in the solid separate. What Compound Is Insoluble In Water.
From rayb78.github.io
Solubility In Water Chart What Compound Is Insoluble In Water substances that dissolve in water to yield ions are called electrolytes. You will see in the. for example, ethanol is soluble in water. The compounds are dissolved in. our article on solubility explains how ionic compounds dissolve in water to form a solution. when ionic compounds dissolve in water, the ions in the solid separate and. What Compound Is Insoluble In Water.
From dxonxarcb.blob.core.windows.net
What Type Of Substances Will Dissolve In Water To Form Aqueous What Compound Is Insoluble In Water You will see in the. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. substances that dissolve in water to yield ions are called electrolytes. However, to predict whether the compound will dissolve or not, we need to. our article on solubility explains how ionic compounds dissolve. What Compound Is Insoluble In Water.
From brentongokemckay.blogspot.com
What Happens When an Ionic Compound Dissolves in Water What Compound Is Insoluble In Water our article on solubility explains how ionic compounds dissolve in water to form a solution. while many compounds are partially or mostly insoluble, there is no substance that is completely insoluble in water, meaning that it can't dissolve at all. Nonelectrolytes are substances that do not produce ions when. In insoluble chemical does not dissolve in the solvent.. What Compound Is Insoluble In Water.
From brainly.com
Which of these compounds are Insoluble in water What Compound Is Insoluble In Water while many compounds are partially or mostly insoluble, there is no substance that is completely insoluble in water, meaning that it can't dissolve at all. our article on solubility explains how ionic compounds dissolve in water to form a solution. Nonelectrolytes are substances that do not produce ions when. However, to predict whether the compound will dissolve or. What Compound Is Insoluble In Water.
From casegrocooley.blogspot.com
Determine Whether Each Compound Is Soluble or Insoluble in Water What Compound Is Insoluble In Water The compounds are dissolved in. Nonelectrolytes are substances that do not produce ions when. However, to predict whether the compound will dissolve or not, we need to. our article on solubility explains how ionic compounds dissolve in water to form a solution. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout. What Compound Is Insoluble In Water.
From www.chegg.com
Solved Solubility rules for ionic compounds in water are What Compound Is Insoluble In Water Nonelectrolytes are substances that do not produce ions when. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. for example, ethanol is soluble in water. You will see in the. while many compounds are partially or mostly insoluble, there is no substance that is completely insoluble in. What Compound Is Insoluble In Water.
From www.thoughtco.com
What Does Insoluble Mean? What Compound Is Insoluble In Water our article on solubility explains how ionic compounds dissolve in water to form a solution. substances that dissolve in water to yield ions are called electrolytes. You will see in the. The compounds are dissolved in. Nonelectrolytes are substances that do not produce ions when. In insoluble chemical does not dissolve in the solvent. when ionic compounds. What Compound Is Insoluble In Water.